Director of Scientific Research
CarbonDrop, founded by a combination of Silicon Valley veterans and genetic engineers, is the most ambitious carbon sequestration company conceived to date, and our aim is to put a serious dent in the climate problems afflicting the planet.
The company’s goal is to sequester tens of gigatonnes of CO2 per year, and thus turn the clock back on global warming. Moreover, given the scalability of the biological innovations we intend to develop, we believe we can accomplish this at a cost per tonne that is radically lower than the achievable targets of the other known carbon dioxide removal companies operating today.
In summary, our goal is to remove more CO2 from the atmosphere each year than is currently being emitted, and to do this at a cost per tonne of less than $5.00, which is more than an order of magnitude lower than competing proposals.
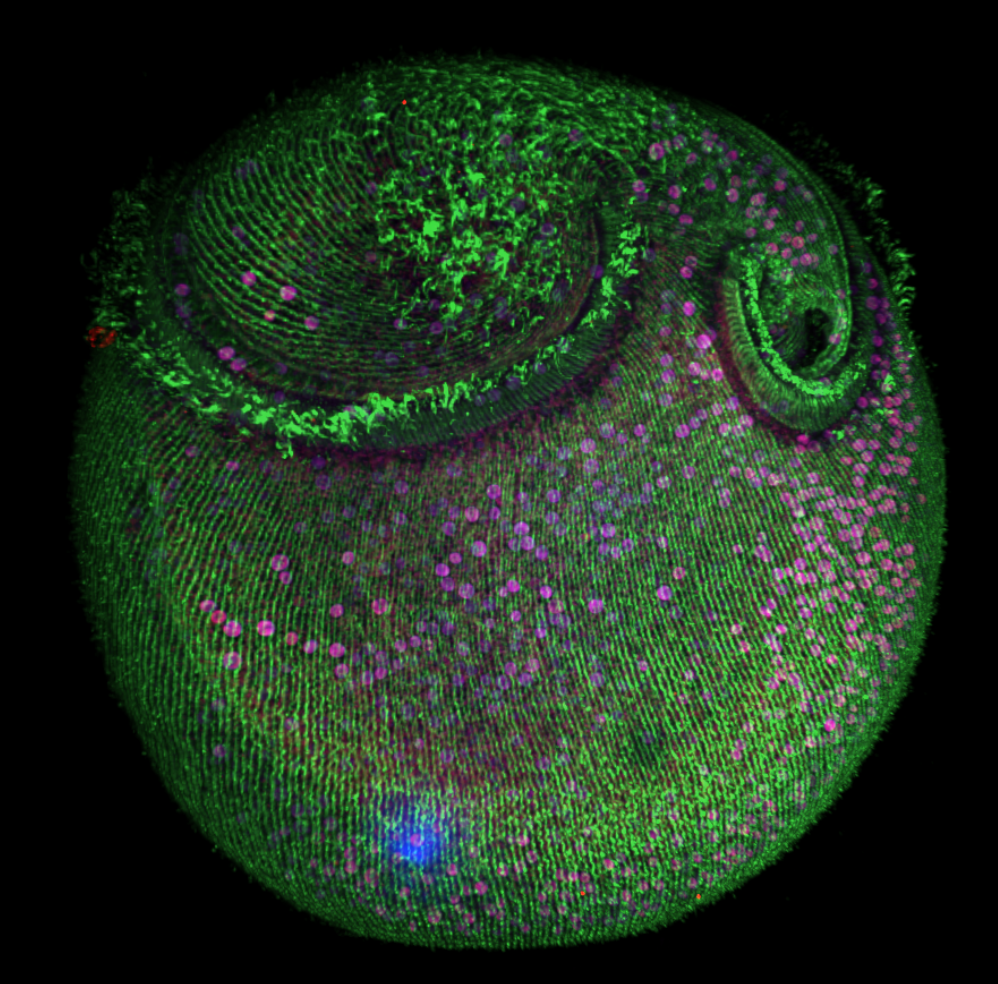
Structural, metabolic and behavioral relationship between a regenerative ciliate and its endosymbiotic algae
Using a wild species of the large and regenerative unicellular ciliate, Stentor, we are studying the structural, metabolic and behavioural relationship between the ciliate and its algal endosymbionts. We are currently developing this Stentor species as a model system for studying algal symbioses and how these symbioses affect the photosynthetic efficiency. This project was started in the context of the Physiology Course at the Marine Biological Laboratory (MBL) and is now being pursued through the MBL's Whitman Center.

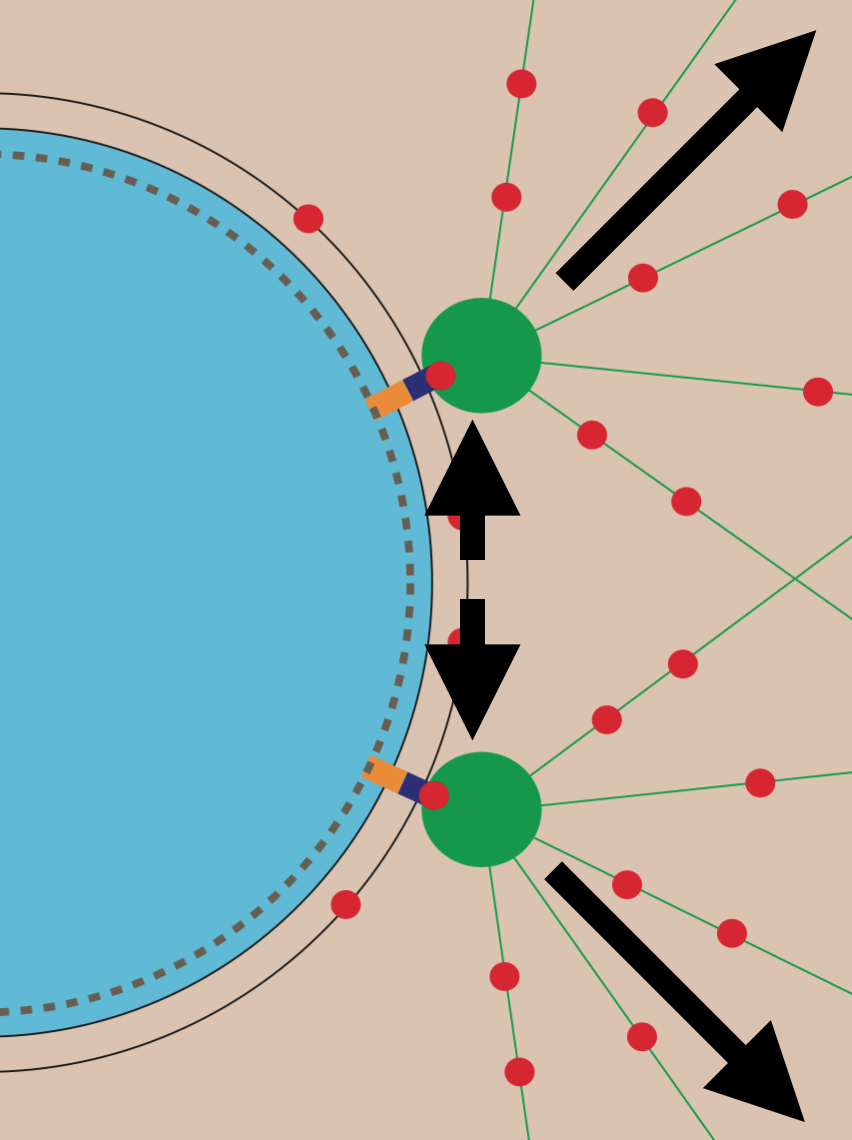
How nuclei expand to form functional organelles
Through a highly collaborative effort between our lab and the labs of Jay Gatlin, Jun Allard and Hernan Garcia, we used quantitative live-cell microscopy assays, mathematical modelling and several model systems to determine how the nucleus is assembled into a functional organelle. Using our model to predict nuclear assembly regulators, we uncovered a role for nucleo-cytoplasmic trafficking in modulating the amount of factors regulating nuclear surface area and hence, nuclear surface tension.
Publication
Boudreau V., Hazel J., Sellinger J.K., Chen P., Manakova K., Radzyminski R., Garcia H.G., Allard J., Gatlin J., Maddox P.S. (2018) Nucleo-cytoplasmic trafficking regulates nuclear surface area during nuclear organogenesis. bioRxiv 326140; doi: https://doi.org/10.1101/326140 (in revision).
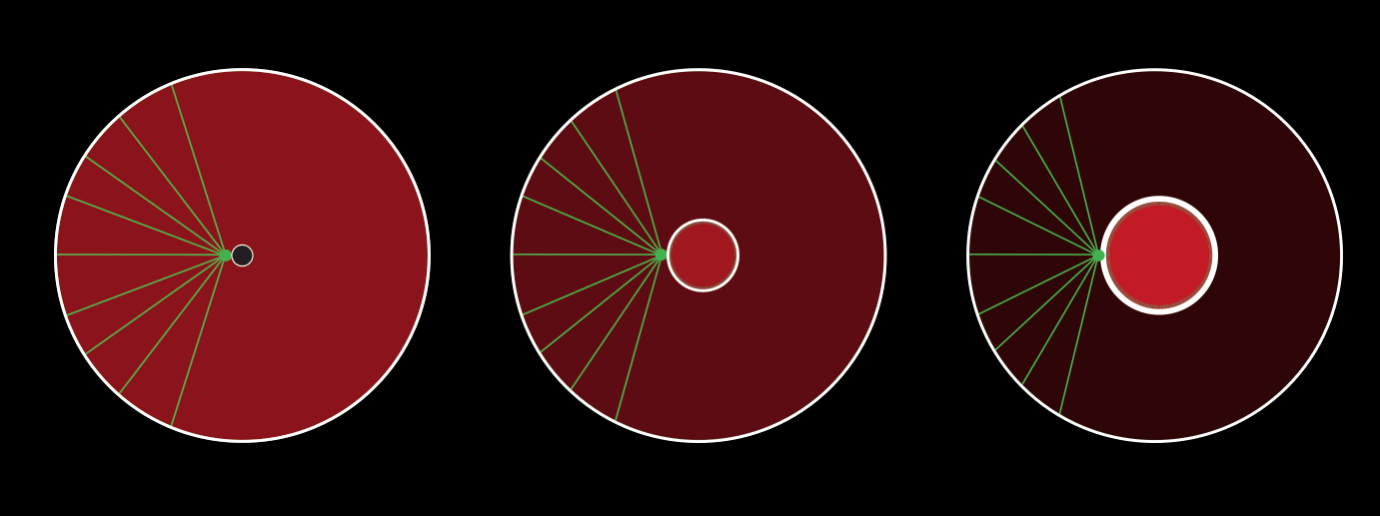
Regulation of centrosome separation during mitotic spindle formation
Having identified a functional interaction between a cell cycle phosphatase and a nuclear lamina component, we investigated the role for these components in regulating early mitotic events in the developing worm embryo. We uncovered a role for the phosphatase and the nuclear lamina in regulating centrosome separation and positioning during mitotic spindle formation of the first embryonic mitosis. In addition to its role in regulation centrosome separation, we also uncovered a role for this phosphatase in regulating Dynein-mediated cortical force generation.
Publications
Boudreau V., Chen R., Edwards A., Muhammad S., Maddox P.S. (2019) PP2A-B55/SUR-6 collaborates with the nuclear lamina for centrosome separation during mitotic entry. Mol Biol Cell; doi: 10.1091/mbc.E18-10-0631. Featured in MBoC's Fifth Annual Special Issue on Quantitative Cell Biology.
Edwards, A., Linehan, J.B., Maddox P.S. & Boudreau V. (2021) Single-particle tracking of dynein identifies PP2A B55/SUR-6 as a cell cycle regulator of cortical force generation. bioRxiv; doi: 10.1101/2021.10.22.465443 (in revision).
Publications
Research articles
Boudreau V.*, Albright A.R.*, Larson B.T., Gerbich T.M., Fadero T., Yan V., Lucas-DeMott A., Yung J., Moulin S.L.Y., Patiño Descovich C., Slabodnick M.M., Burlacot A., Wang J.R., Niyogi K.K. & Marshall W.F. (2025) The cell biology and genome of Stentor pyriformis, a giant cell that embeds symbiotic algae in a microtubule meshwork. MBoC; doi: 10.1091/mbc.E24-12-0571. Featured in MBoC's Special Issue on Evolutionary Cell Biology.
Linehan J.B., Edwards G.A., Boudreau V., Maddox A.S. & Maddox P.S. (2023) Model-based trajectory classification of anchored molecular motor-biopolymer interactions. Biophys. Rep.; doi: 10.1101/2021.10.22.465443.
Edwards A., Linehan J.B., Maddox P.S. & Boudreau V. (2021) Single-particle tracking of dynein identifies PP2A B55/SUR-6 as a cell cycle regulator of cortical force generation. bioRxiv; doi: 10.1101/2021.10.22.465443 (in revision).
Dumont M.*, Gamba R.*, Gestraud P, Klaasen S, Worrall J.T., De Vries S.G., Boudreau V., Maddox P.S., Lens S.M.A., Kops G.J.P.L., Mc Clelland S., Miga K.H. & Fachinetti D. (2019) Human chromosome-specific aneuploidy is influenced by DNA-dependent centromeric features. EMBO J; doi: 10.15252/embj.2019102924 *equal contribution.
Boudreau V., Chen R., Edwards A., Muhammad S., Maddox P.S. (2019) PP2A-B55/SUR-6 collaborates with the nuclear lamina for centrosome separation during mitotic entry. Mol Biol Cell; doi: 10.1091/mbc.E18-10-0631. Featured in MBoC's Fifth Annual Special Issue on Quantitative Cell Biology.
Hatkevich T., Boudreau V., Rubin T., Huynh J.-R., Maddox P.S., Sekelsky J. (2019) Centromeric SMC1 promotes centromere clustering and stabilizes meiotic homolog pairing. PLoS Genetics; doi: 10.1371/journal.pgen.1008412.
Mehsen M., Boudreau V., Garrido D., Bourouh M., Larouche M., Maddox P.S., Swan A., Archambault V. (2018) PP2A-B55 promotes nuclear envelope reformation after mitosis in Drosophila. J Cell Biology; doi: 10.1083/jcb.201804018.
Byrnes A.E., Lowe B.F., Boudreau V., Slep K.C. Polarized TOG arrays cooperatively bind tubulin to promote microtubule dynamics (in revision).
Boudreau V., Hazel J., Sellinger J.K., Chen P., Manakova K., Radzyminski R., Garcia H.G., Allard J., Gatlin J., Maddox P.S. (2018) Nucleo-cytoplasmic trafficking regulates nuclear surface area during nuclear organogenesis. bioRxiv 326140; doi: 10.1101/326140 (in revision).
Science policy with the Future of Research (FoR)
Bankston, A., Davis, S. M., Moore, E., Niziolek, C. A., Boudreau V. (2020) Research Culture: Why scientific societies should involve more early-career researchers. eLife; doi: 10.7554/eLife.60829.Methods/protocols/chapters
Ryan J., Gerhold A.R., Boudreau V., Smith L., Maddox P.S. (2017) Introduction to Modern Methods in Light Microscopy. In: Markaki Y., Harz H. (eds) Light Microscopy. Methods in Molecular Biology, vol 1563. Humana Press, New York, NY; doi: 10.1007/978-1-4939-6810-7_1.
Other
Cellular Collaboration: Whitman Fellow Shines Light on Unique Symbiosis.